New Research Shows Virus Plays Ultimate Game of ‘Hide and Seek’ with Immune System
SARS-CoV-2-infected individuals could have different variants hidden in different parts of the body.
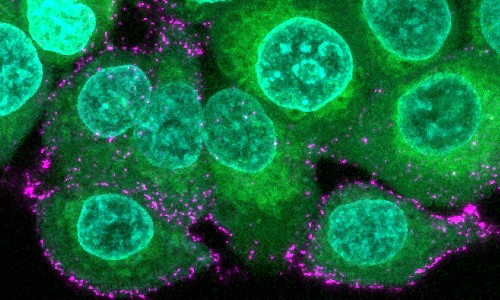
Image of human epithelial cells (green with blue nuclei) are incubated with synthetic SARS-CoV-2 virions (magenta) to study infection and immune evasion.
Image credit: Oskar Staufer and MPI for Medical Research, Germany
SARS-CoV-2-infected individuals could have different variants hidden in different parts of the body.
People suffering from COVID-19 could have several different SARS-CoV-2 variants hidden away from the immune system in different parts of the body, finds new research published in Nature Communications by an international research team. The study’s authors say that this may make complete clearance of the virus from the body of an infected person, by their own antibodies, or by therapeutic antibody treatments, much more difficult.
COVID-19 continues to sweep the globe causing hospitalisations and deaths, damaging communities and economies worldwide. Successive variants of concern (VoC), replaced the original virus from Wuhan, increasingly escaping immune protection offered by vaccination or antibody treatments.
In new research, comprising two studies published in parallel in Nature Communications, an international team led by Professor Imre Berger at the University of Bristol and Professor Joachim Spatz at the Max Planck Institute for Medical Research in Heidelberg , both Directors of the Max Planck Bristol Centre of Minimal Biology, show how the virus can evolve distinctly in different cell types, and adapt its immunity, in the same infected host.
Spike protein structure from BrisDelta, an early SARS-CoV-2 variant discovered in Bristol. Credit: Christine Toelzer, University of Bristol.
The team sought to investigate the function of a tailor-made pocket in the SARS-CoV-2 spike protein in the infection cycle of the virus. The pocket, discovered by the Bristol team in an earlier breakthrough, played an essential role in viral infectivity.
“An incessant series of variants have completely replaced the original virus by now, with Omicron and Omicron 2 dominating worldwide.” said Professor Imre Berger. “We analysed an early variant discovered in Bristol, BrisDelta. It had changed its shape from the original virus, but the pocket we had discovered was there, unaltered”. Intriguingly, BrisDelta, presents as a small subpopulation in the samples taken from patients, but appears to infect certain cell-types better than the virus that dominated the first wave of infections.
Dr Kapil Gupta, lead author of the BrisDelta study, explains: “Our results showed that one can have several different virus variants in one’s body. Some of these variants may use kidney or spleen cells as their niche to hide, while the body is busy defending against the dominant virus type. This could make it difficult for the infected patients to get rid of SARS-CoV-2 entirely.”
The team applied cutting-edge synthetic biology techniques, state-of-the-art imaging and cloud computing to decipher viral mechanisms at work. To understand the function of the pocket, the scientists built synthetic SARS-CoV-2 virions in the test tube, that are mimics of the virus but have a major advantage in that they are safe, as they do not multiply in human cells.
Using these artificial virions, they were able to study the exact mechanism of the pocket in viral infection. They demonstrated that upon binding of a fatty acid, the spike protein decorating the virions changed their shape. This switching ‘shape’ mechanism effectively cloaks the virus from the immune system.
Dr Oskar Staufer, lead author of this study and joint member of the Max Planck Institute in Heidelberg and the Max Planck Centre in Bristol, explains: “By ‘ducking down’ of the spike protein upon binding of inflammatory fatty acids, the virus becomes less visible to the immune system. This could be a mechanism to avoid detection by the host and a strong immune response for a longer period of time and increase total infection efficiency.”
“It appears that this pocket, specifically built to recognise these fatty acids, gives SARS-CoV-2 an advantage inside the body of infected people, allowing it to multiply so fast. This could explain why it is there, in all variants, including Omicron” added Professor Berger. “Intriguingly, the same feature also provides us with a unique opportunity to defeat the virus, exactly because it is so conserved - with a tailormade antiviral molecule that blocks the pocket.” Halo Therapeutics, a recent University of Bristol spin-out founded by the authors, pursues exactly this approach to develop pocket-binding pan-coronavirus antivirals.
The team included experts from Bristol UNCOVER Group, the Max Planck Institute for Medical Research in Heidelberg, Germany, Bristol University spin-out Halo Therapeutics Ltd and further collaborators in UK and in Germany. The studies were supported by funds from the Max Planck Gesellschaft, the Wellcome Trust and the European Research Council, with additional support from Oracle for Research for high-performance cloud computing resources. The authors are grateful for the generous support by the Elizabeth Blackwell Institute of the University of Bristol.
Publication: Kapil Gupta, et al., Structural insights in cell-type specific evolution of intra-host diversity by SARS-CoV-2, Nature Communications (2022). DOI: 10.1038/s41467-021-27881-6.
Original Story Source: University of Bristol

 Alerts Sign-up
Alerts Sign-up