Dissociation Mechanism of Oxygen Molecules on a Silver Surface Unveiled
The mechanism by which oxygen molecules break up into atoms on silver surfaces has been clarified for the first time
The mechanism by which oxygen molecules break up into atoms on silver surfaces has been clarified for the first time
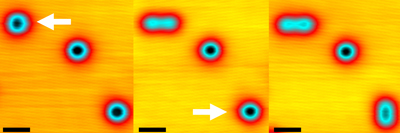 Figure 1: Scanning tunneling microscopy images showing two oxygen molecules on a silver surface being disassociated by the application of a positive voltage pulse. The molecule on the top left is disassociated first (middle image), followed by the one on the bottom right (right image). Adapted, with permission, from Ref. 1. Copyright 2022 American Chemical Society.
Figure 1: Scanning tunneling microscopy images showing two oxygen molecules on a silver surface being disassociated by the application of a positive voltage pulse. The molecule on the top left is disassociated first (middle image), followed by the one on the bottom right (right image). Adapted, with permission, from Ref. 1. Copyright 2022 American Chemical Society.
The intricacies of how a molecule of oxygen (O2) on a silver surface separates into two oxygen atoms have been determined by RIKEN chemists1. This knowledge will help scientists to optimize industrially important reactions.
Many industrial processes use silver surfaces to catalyze the breakup of oxygen molecules into their constituent atoms. But while this reaction has been widely investigated, the exact reaction pathway remained unknown.
“It’s hard to believe we still don’t know how oxygen molecules disassociate on a silver surface. The reaction has been exploited in industry, and its mechanism seems simple,” reflects Minhui Lee of the RIKEN Surface and Interface Science Laboratory. “It’s essential to observe the reaction at a single-molecule level to understand the details.”
To study the dissociation mechanism in detail, Lee, Emiko Kazuma and their co-workers, in a team led by Yousoo Kim, used a scanning tunneling microscope (STM)—an instrument with an atomically sharp tip that is brought close enough to a sample to allow electrons to tunnel between the tip and the sample.
Oxygen molecules can absorb onto a silver surface in two configurations. The researchers used the STM to investigate the reactivity for both configurations. They then used the STM tip to inject electrons into the molecule by applying positive voltage pulses, or to inject holes with negative voltage pulses. Both processes broke the oxygen–oxygen bond and resulted in two separate oxygen atoms on the surface.
Studying how the dissociation yield depends on the energy of electrons/holes sheds light on the excitation channels at work. Two sequential excitations were needed to overcome the relatively high reaction barrier, and the molecules were excited to higher-order vibrational excited states before dissociating.
“The reaction pathway is an excitation from the ground state to higher excitations of the vibrational states, namely overtones; in our case, higher than the fifth excited state,” explains Lee. “We had to consider the deviation of the system from a harmonic oscillator—a commonly used simplification in quantum physics. That wasn’t easy, but after comparing the experimental data with calculations, we were able to identify the overtones involved in the reaction.”
The team plans to take their work further. “In this study, we focused on molecules adsorbed on a metal surface, with the molecular orbitals strongly hybridized with the surface,” concludes Kazuma. “The next step is to investigate molecules slightly decoupled from the surface in order to understand the influence of the metal surface on chemical reactions.”
Publication: Minhui Lee, et al., Dissociation Mechanism of a Single O2 Molecule Chemisorbed on Ag(110), American Chemical Society (2022). DOI: 10.1021/acs.jpclett.1c02456.
Original Story Source: RIKEN

 Alerts Sign-up
Alerts Sign-up