‘Click’ Chemistry May Help Treat Dogs With Bone Cancer, MU Study Finds
In September, researchers from California and Denmark were awarded a Nobel Prize in Chemistry for their development of ‘click’ chemistry, a process in which molecules snap together like LEGO, making them a potentially more efficient transportation device in delivering pharmaceuticals to cancer tumors.
In September, researchers from California and Denmark were awarded a Nobel Prize in Chemistry for their development of ‘click’ chemistry, a process in which molecules snap together like LEGO, making them a potentially more efficient transportation device in delivering pharmaceuticals to cancer tumors.
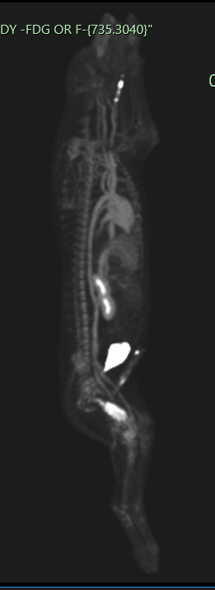 PET scan images of a dog show a cancerous tumor in the femur (thigh bone).
PET scan images of a dog show a cancerous tumor in the femur (thigh bone).
Now, in a recent study, a researcher at the University of Missouri has successfully shown for the first time how click chemistry can be used to more efficiently deliver drugs to treat tumors in large dogs with bone cancer – a process that had previously only been successful in small mice.
“If you want to attack a tumor using the immune system, an antibody is an extremely specific way to deliver a drug or radioactive payload to the tumor, but the problem with antibodies is they are huge molecules that circulate in the bloodstream for days or even weeks,” said Jeffrey Bryan, a professor in the MU College of Veterinary Medicine and author on the study. “If you put a drug or radioactive molecule onto the antibody, you leave radioactivity circulating in the bloodstream for a long time, which can spread to and negatively impact organs, bone marrow and the liver while not getting as much dose to the specific tumor as you were hoping for.”
The goal with click chemistry is to maximize the delivery of therapeutic drugs specifically to the cancer tumor to increase effectiveness while minimizing the circulation of those drugs throughout the bloodstream, which may cause dangerous side effects.
From mice to man’s best friend
For years, many chemists assumed that while click chemistry has been successful in mice, the strategy would not work in large dogs or people because the size of the body might be too big for the two sides of therapy-delivering molecules to find each other and snap, or ‘click,’ together. Bryan collaborated with Brian Zeglis, an associate professor at Hunter College in New York who specializes in click chemistry, to conduct the first-ever successful ‘proof-of-concept’ study at the MU College of Veterinary Medicine. Using click chemistry, doses of radiopharmaceuticals were delivered specifically to the tumors in five dogs that weighed more than 100 pounds and had bone cancer.
“It is a huge step forward for the field to show that this worked in a human-sized body,” Bryan said. “Going forward, this may pave the way for click chemistry to be used to help humans with cancer in the future.”
Bryan has been researching veterinary and comparative oncology for nearly two decades. He said some dogs with one known bone tumor have additional bone tumors hiding in their body’s skeleton. An additional benefit of studies involving imaging scans and click chemistry is the ability to discover if additional cancer tumors are located in a dog’s skeleton and impacting their health.
“Osteosarcoma, a common form of bone cancer, impacts both dogs and people, and it causes severe pain, limping, swelling in the limbs, and treating the bone tumors with various radiation therapy and immune therapy approaches to take away the pain is something I am passionate about here at MU," Bryan said. "Everything we learn about treating these dogs can be translated to help humans down the road.”
A leader in treating cancer – for people and pets
The MU College of Veterinary Medicine, which earned more than $14 million in federal research funding last year from the National Institutes of Health, is the site of clinical trials for cancer that attract people and their pets from California, Florida, New York and states across the country.
“It is heartwarming to be a part of it because the patients’ families realize it is not just about better outcomes for their specific dog, but they are also contributing to better outcomes for other dogs in the future and hopefully better health outcomes for people as we translate these advances from the dogs to the human side,” Bryan said.
 Jeffrey Bryan is a professor in the MU College of Veterinary Medicine.
Jeffrey Bryan is a professor in the MU College of Veterinary Medicine.
While this was a successful ‘proof-of-concept’ imaging study involving click chemistry, Bryan’s long-term goal is to develop a therapy using radiopharmaceuticals, potentially involving an antibody-targeting molecule, to treat dogs with bone cancer that may not be well enough for other treatments that involve surgery.
“This research is also an example of precision medicine, a key part of MU’s NextGen Precision Health initiative, because we are using the molecules associated with the specific tumor to deliver the therapeutic dose of treatment,” Bryan said. “We collaborate with the MU Research Reactor, the Molecular Imaging and Theranostics Center, and Washington University in St. Louis, so it is a team effort.”
In 2020, Bryan collaborated with ELIAS Animal Health to create a precision medicine approach – a vaccine from a dog’s own tumor – to target and kill cancer cells in dogs suffering from osteosarcoma. The success of the treatment in dogs led the Food and Drug Administration to grant a rare fast-track designation for ELIAS Animal Health’s parent organization, TVAX Biomedical, to study the ELIAS immunotherapy approach to treat glioblastoma multiforme, a cancerous brain tumor in humans.
“The last dog that participated in that study just died a few weeks ago, five years out from their original diagnosis of bone cancer, and the dog never relapsed with its cancer, so the dog was able to live the rest of its life cancer-free due to the immunotherapy,” Bryan said. “Our overall goal is to come up with different tools in our toolbox to effectively help treat dogs with cancer, and one day even people, too.”
“Pretargeted PET of Osteodestructive Lesions in Dogs” was published in Molecular Pharmaceutics. Funding was provided by Hunter College.
Publication: Charles A. Maitz, et al., Pretargeted PET of Osteodestructive Lesions in Dogs, ACS Publications (2022). DOI: 10.1021/acs.molpharmaceut.2c00220
Original Story Source: University of Missouri

 Alerts Sign-up
Alerts Sign-up