Down to the Synapse: Connecting Brain Circuits to Behavior
When a threat is looming and an escape route is open, one would expect any animal to flee imminent danger.
When a threat is looming and an escape route is open, one would expect any animal to flee imminent danger. But when microscopic worms faced a threatening signal in a lab at the Weizmann Institute of Science, only the females fled right away. The males stayed put until the signal grew much stronger. Puzzled at this difference between the sexes, the scientists used it to explore one of the greatest enigmas surrounding the brain: how the structure of its neural circuits shapes behavior.
The transparent, one-millimeter-long worm, Caenorhabditis elegans, is uniquely suited for probing this question because it has a relatively broad repertoire of behaviors, and the wiring of its nervous system, consisting of only some 300 neurons, has been mapped for both sexes. “We wanted to find out how the neural networks encode such different behaviors in females and males,” says Dr. Meital Oren-Suissa, whose lab in Weizmann’s Brain Sciences Department studies sex-related differences in the brain and the rest of the nervous system, mainly using C. elegans as a model.
 (l-r) Prof. Elad Schneidman, Vladyslava (Lada) Pechuk, Dr. Yehuda Salzberg, Gal Goldman and Dr. Meital Oren-Suissa
(l-r) Prof. Elad Schneidman, Vladyslava (Lada) Pechuk, Dr. Yehuda Salzberg, Gal Goldman and Dr. Meital Oren-Suissa
In experiments led by graduate student Vladyslava (Lada) Pechuk, the scientists found that worms exposed to harmful chemical substances sense the danger using receptors similar to those that convey the sensation of pain in humans, and the response of these receptors to the dangerous cue is similar in worms of both sexes. The researchers also found that while the neural circuit that picks up the danger signal consists of the same neurons in females and males, the wiring of these neurons – that is, how they are connected to one another – differs in several ways between the sexes. The big unknown was which of these differences matter in terms of behavior.
In response to a dangerous cue, the female (left) flees right away, whereas the male (right) stays put. The female is larger than the male, she is actually a hermaphrodite: Her reproductive organs produce both eggs and sperm
To address this question, Oren-Suissa teamed up with departmental colleague Prof. Elad Schneidman, whose areas of study include the relations between the architecture of neural networks and their function. Gal Goldman, a student under the joint supervision of Oren-Suissa and Schneidman, simulated the danger-sensing circuits of females and males, using mathematical models that she fitted to experimental data so that the simulations would recapitulate the recorded neural activity and the worms’ behavioral responses. Her model predicted that the differences in the wiring of the circuits in the two sexes would be sufficient to generate the different behaviors. She and the team then proceeded to the next challenge: determining which parts of the circuits are responsible for the behavioral difference. Moreover, they used the model to predict how changing the wiring might change the circuits’ function.
"We managed to reprogram their behavior just by altering a single connection in their neural circuitry"
The model identified a critical difference in the wiring, which boiled down to a single connection, or synapse, between two neurons. These two neurons were connected to one another in the female, but not the male, worms.
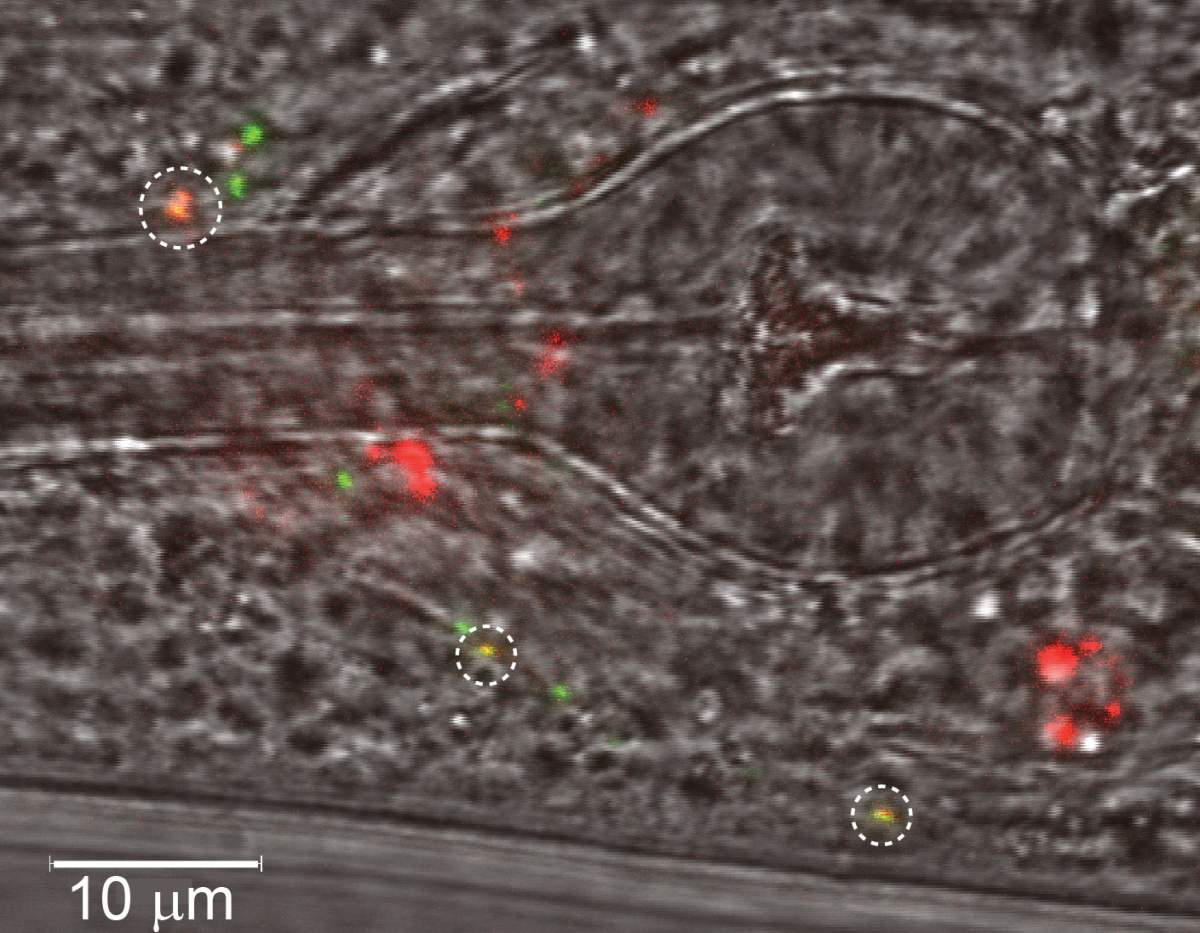 Part of a male worm’s head under a microscope, with artificially added synapses marked by circles
Part of a male worm’s head under a microscope, with artificially added synapses marked by circles
“It’s widely assumed that the connections between neurons determine what a brain circuit can do, but it’s usually hard to test this experimentally in complex systems such as the mammalian brain,” Schneidman explains. “The worm’s nervous system, in contrast, is small, relatively simple and experimentally accessible, so after making computational predictions about its wiring, we could ‘reprogram’ the circuit, thus putting our predictions to the test in a real animal.”
In a feat of molecular engineering, Pechuk and colleagues inserted the missing connection into the danger-sensing circuit of male worms, so that it would mimic that of females. As the model had predicted, the males equipped with the synthetic synapse started fleeing from the danger, just like the females.
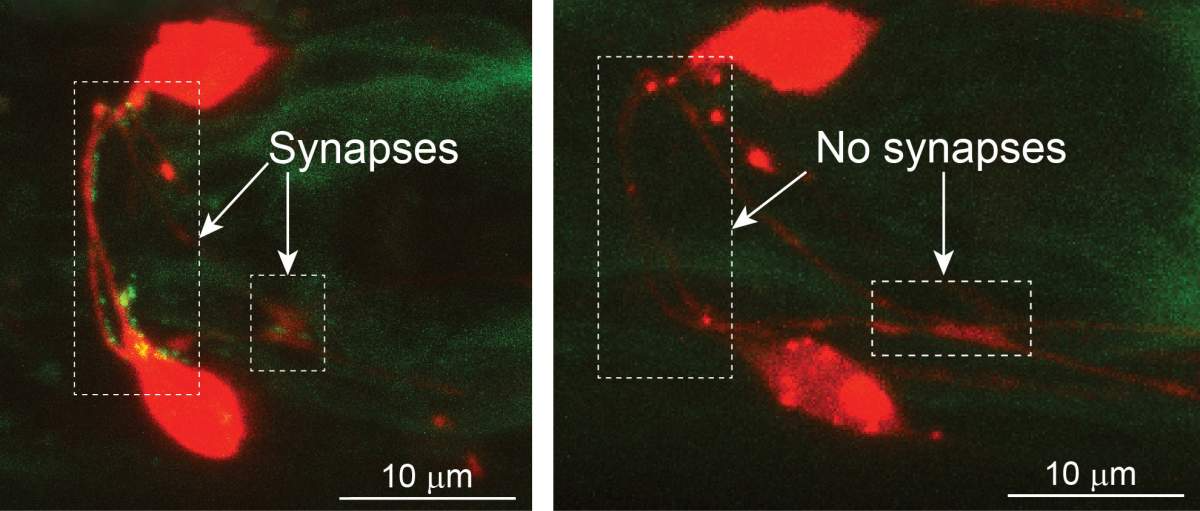 Neurons (red) in the worm’s danger-sensing neural circuits. Fluorescent labeling reveals active neuronal connections (bright-green dots) that are present in females (left) but not in males
Neurons (red) in the worm’s danger-sensing neural circuits. Fluorescent labeling reveals active neuronal connections (bright-green dots) that are present in females (left) but not in males
“We managed to reprogram their behavior just by altering a single connection in their neural circuitry,” Oren-Suissa says.
Why were the unaltered male worms so prone to risk-taking? The researchers believe that this may be due to the evolutionary force that Darwin termed “sexual selection”: the preservation of features that help the animal find mates at the cost of creating greater exposure to predators or other dangers – a peacock’s tail being one iconic example. In the case of C. elegans, the researchers hypothesized that if males possessed the ability to respond to danger or pain, they would be less competent in finding a mate.
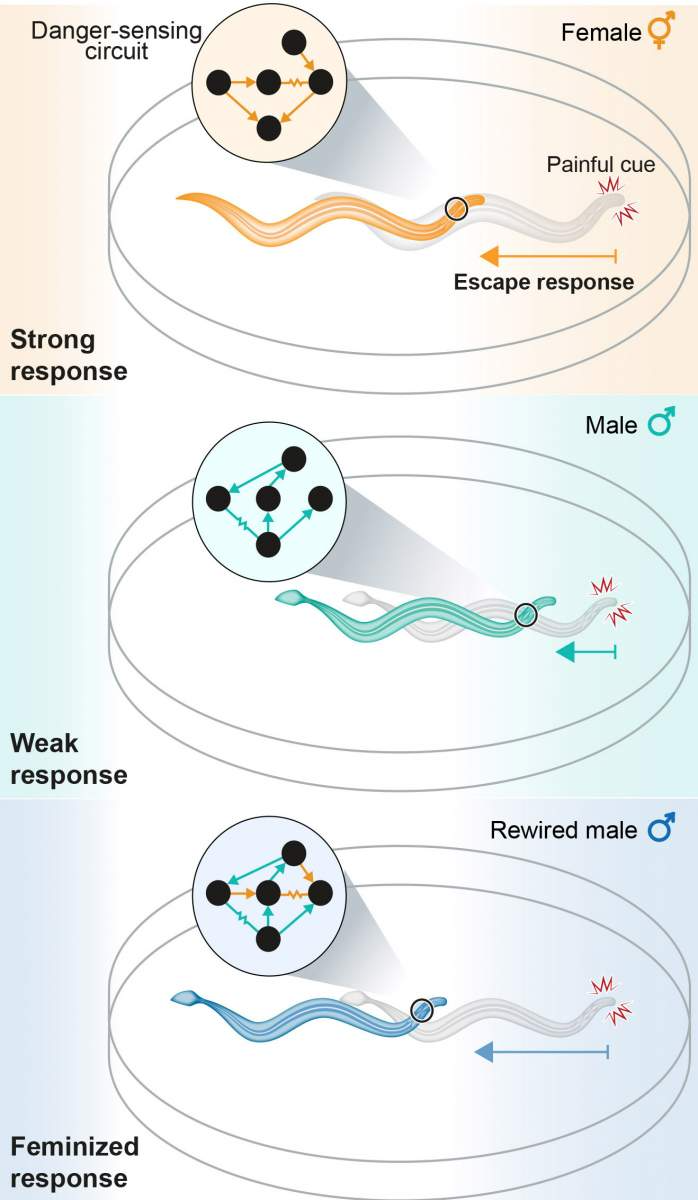 A drawing showing the danger-sensing neural circuit in female (top) and male (middle) worms. When a connection was added to make the circuit of males resemble that of females (bottom), the males responded to the dangerous cue similarly to females
A drawing showing the danger-sensing neural circuit in female (top) and male (middle) worms. When a connection was added to make the circuit of males resemble that of females (bottom), the males responded to the dangerous cue similarly to females
“The male worm has to take risks – if he avoids all worrisome signals in his environment, he may never get to the female,” Oren-Suissa explains. This is in contrast to the female, which is actually a hermaphrodite: Her reproductive organs produce both eggs and sperm, so she can be inseminated either by her own sperm or by that of a male.
This explanation was borne out by experiments. Whereas regular males rushed directly to the females – danger be damned – the engineered males with a synthetic synapse were much more hesitant, taking ten times longer to reach potential mates.
C. elegans takes 3 days to mature from an egg into an adult organism. It lives for about 3 weeks in the lab but can survive for months in its natural habitat of fruit rotting in soil. Even though it lacks eyes and ears, it responds to light and airborne sound. It is capable of learning and can retain memories for about 1 day – not bad even by human standards.
The study’s findings vividly illustrate the notion that altering synapses – which retain the same basic architecture from worms to humans – can lead to behavioral changes. Obviously, the human brain, with its trillions of synapses, compared to the worm’s 5,000, is vastly more complex than the nervous system of C. elegans. “Flipping one synapse in humans won’t change their behavior – that would probably require rewiring on an entirely different scale,” Schneidman says. But the study’s findings shed new light on the way the layout of neural circuits in the brain translates into behavioral tendencies and traits. In the future these findings may prove relevant to building artificial systems that mimic the entire human brain or its parts. The study also suggests a new direction for investigating why the two sexes in higher species, including humans, perceive pain differently.
Assistant Staff Scientist Dr. Yehuda Salzberg from Oren-Suissa’s lab took part in the research. Also participating in the study were scientists from the State University of New York at Buffalo and from the University of Rochester.
Publication: Vladyslava Pechuk, et al., Reprogramming The Topology Of The Nociceptive Circuit In C. elegans Reshapes Sexual Behavior, Science Direct (2022). DOI: 10.1016/j.cub.2022.08.038
Original Story Source: Weizmann Institute of Science

 Alerts Sign-up
Alerts Sign-up