Life’s Mysteries Converge in a Droplet
Recreating conditions that may have existed before the dawn of life, researchers watched droplets give rise to possible precursors of today’s proteins.
Two biological mysteries, set billions of years apart, have come together in a recent research project at the Weizmann Institute of Science.
One has to do with the origin of life, or more precisely, the origin of proteins – those biological molecules essential for life. How did small segments of proteins floating in the primordial soup – the wet, organic-molecule rich environment believed to have existed on Earth some four billion years ago – evolve into the much larger, more complex protein molecules we know today?
 (l-r) Dr. Manas Seal and Prof. Daniella Goldfarb
(l-r) Dr. Manas Seal and Prof. Daniella Goldfarb
Seeking to address this question, scientists in the lab of the late Prof. Dan Tawfik, together with colleagues, mathematically reconstructed a possible long-lost ancestor of modern proteins. In their model this ancestor consisted of two small protein segments, or peptides, with nearly identical sequences of amino acids – a peptide “word” repeated twice to make a simple protein “phrase.” This repetition suggested that some of the first proteins might have been created through the pairing up of amino acid sequences.
"It breaks my heart that Danny never got to see this result,” Longo says. “He so much wanted to see these dimers"
To reproduce this process in the lab, the scientists synthesized their hypothetical ancestor protein, cut it in two and then placed the resulting peptide “words” in water. They could find no sign of the words assembling into pairs, but when Dr. Liam Longo, then a postdoctoral fellow in Tawfik’s lab, added to the mix a certain amount of RNA – which may have been a dominant molecule in the prelife world – the water suddenly turned cloudy. Under the microscope, Longo saw that the positively charged peptides had condensed into droplets as they combined with the negatively charged RNA.
“This was really exciting because the assembly events we’d been looking for could have taken place within such droplets,” says Longo, now a principal investigator at the Tokyo Institute of Technology.
 Tiny droplets formed by peptides and RNA, viewed under a microscope
Tiny droplets formed by peptides and RNA, viewed under a microscope
In fact, just over a decade ago scientists had discovered that similar droplets are naturally present inside cells. These fascinating constructs are liquid-like assemblies of proteins alone, or proteins with RNA or DNA; they form something like oil drops in water, through phase separation between two liquids. Inside the droplets, proteins may reach concentrations that are hundreds of times higher than they are outside. In 2018, Science magazine nominated droplets for the Breakthrough of the Year, referring to the study of such droplets as “one of the hottest topics in cell biology.” Yet much is still unknown about the mechanisms by which these droplets form and what purposes they serve in cells – for example, might they be used for storage or for performing regulatory functions.
That’s where the two biological mysteries converge. Tawfik, of Weizmann’s Biomolecular Sciences Department, undertook the study of peptide-RNA droplets to see whether they might have played a role in the origin of proteins, but it was clear that this research could also shed new light on the roles that similar droplets play today in the human body.
The dimer hunt
Tawfik and colleagues hypothesized that in the prelife world, the peptide-RNA droplets formed in the primordial soup may have served as protocells, forerunners of modern living cells. The droplets would have created a kind of compartment, bringing peptides close together from their isolation in the vast, dilute soup, thereby enabling them to self-assemble into dual structures called dimers: pairs of peptide molecules drawn to one another by molecular forces. A dimer might later fuse into a single molecule, creating an ancestral two-word protein akin to the one reconstructed by the scientists.
Finding evidence for the existence of such dimers, however, was problematic because they could not be detected by standard structure-solving methods that apply to solutions: The resolution provided by these methods is too low for this task. To overcome this hurdle, Tawfik and colleagues teamed up with Prof. Daniella Goldfarb of Weizmann’s Chemical and Biological Physics Department, who studies the structure and dynamics of proteins by means of electron paramagnetic resonance. “This collaboration grew out of my friendship with Danny,” Goldfarb recalls. “He told me that he had a hypothesis about the evolution of complex proteins from primordial peptides, and I suggested testing this idea experimentally, using the expertise of my lab.”
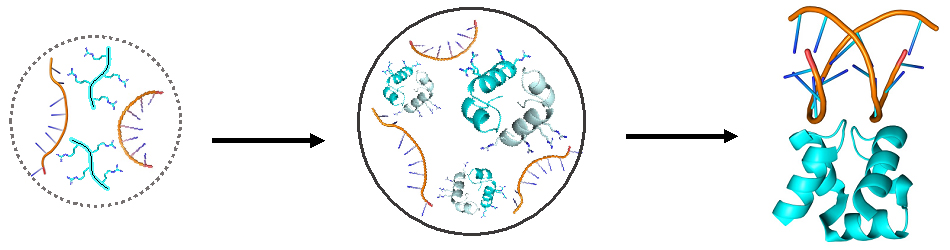 Proposed evolution of ancient proteins: from individual structureless free-floating peptides (turquoise, left) to a fused and structured DNA-binding ancestral protein (turquoise, right) via an intermediate step – peptides assembled into dimers inside droplets (middle)
Proposed evolution of ancient proteins: from individual structureless free-floating peptides (turquoise, left) to a fused and structured DNA-binding ancestral protein (turquoise, right) via an intermediate step – peptides assembled into dimers inside droplets (middle)
Goldfarb and colleagues set out to look for Tawfik’s proposed dimers by tagging the peptides with spin labels, tiny molecules having special magnetic properties, prepared in the lab of Prof. Norman Metanis at the Hebrew University of Jerusalem. When the tagged peptides are then placed in a magnetic field, the tags can act as spies that report on the properties of the peptide to which they are attached. Thus, when the scientists performed an experiment called double electron-electron resonance, in which they applied a series of microwave pulses to a solution containing peptides, they could tell how far the peptides were from one another by picking up on certain magnetic interactions between the tags. Should the interactions reveal that two peptides are really close, this would mean that they had formed a dimer. But optimizing experimental conditions was tricky, and the initial measurements failed to spot any dimers in a solution of peptides, with or without RNA.
The tragedy and the triumph
In the middle of the search, tragedy struck. On May 4, 2021, Tawfik fell to his death in a rock-climbing accident.
With uncanny timing, the following day Dr. Manas Seal, a postdoctoral fellow in Goldfarb’s lab, managed to find dimers in a peptide solution.
“It breaks my heart that Danny never got to see this result,” Longo says. “He so much wanted to see these dimers.”
 The late Prof. Dan Tawfik. Searching for the origin of life
The late Prof. Dan Tawfik. Searching for the origin of life
The scientists carried on with their experiments to see whether peptides also form dimers when they are bound to RNA within droplets. First, the researchers proved that the peptides do indeed bind to RNA in the droplets: They confirmed this by detecting a slowing down in the motion of their spies. Next, they set out to trace magnetic interactions among the spies. Interpreting magnetic signals obtained from densely packed droplets, however, was extremely difficult. The scientists analyzed a wide variety of different ratios of RNA and peptides till they managed to prove the existence of the dimers within the droplets.
“We found that the binding to the RNA actually enhances the formation of the dimers,” says Seal, who led these experiments. “This binding probably helps the peptides find one another, just as two people separated by a great distance could find each other more easily if they hold on to the same rope.”
 Dr. Liam Longo
Dr. Liam Longo
The study’s findings suggest that in the ancient past, droplets might have been crucibles for a critical step in protein evolution. They might have made possible the transition from short peptides to longer proteins that fold into the intricate structures enabling them to perform biological functions. One of the first such functions – the one seen in the ancestral protein reconstructed by Tawfik and colleagues – was to bind to nucleic acids, such as RNA or DNA, a necessary first step in promoting the expression of the genetic code. “In our paper, we refer to droplets as a ‘cradle’ for the evolution of folded proteins,” Longo says.
Science Numbers
Proteins found in droplets within our cells can reach concentrations of 3 to 15 micromolar – hundreds of times higher than in the cytoplasm.
These insights might also prove relevant for understanding what happens inside the droplets that are involved in a variety of fundamental cellular processes in both the healthy and the diseased human body. For example, defects in the mechanisms of droplet formation are thought to contribute to neurodegenerative diseases; an in-depth knowledge of these mechanisms, and of the ways in which they affect protein structure, may point toward new diagnostic methods and treatments.
Study authors also included Dr. Dragana Despotovic from Tawfik’s lab; Orit Weil-Ktorza and Prof. Norman Metanis of the Hebrew University of Jerusalem; and Prof. Koby Levy of Weizmann’s Chemical and Structural Biology Department.
Publication: Manas Seal, et al., Peptide-RNA Coacervates as a Cradle for the Evolution of Folded Domains, Journal of the American Chemical Society (2022). DOI: 10.1021/jacs.2c03819
Original Story Source: Weizmann Institute of Science

 Alerts Sign-up
Alerts Sign-up