This Is How An Alzheimer’s Gene Ravages The Brain
Study in cells and mice suggests that the variant APOE4 affects the all-important insulation around nerve cells.
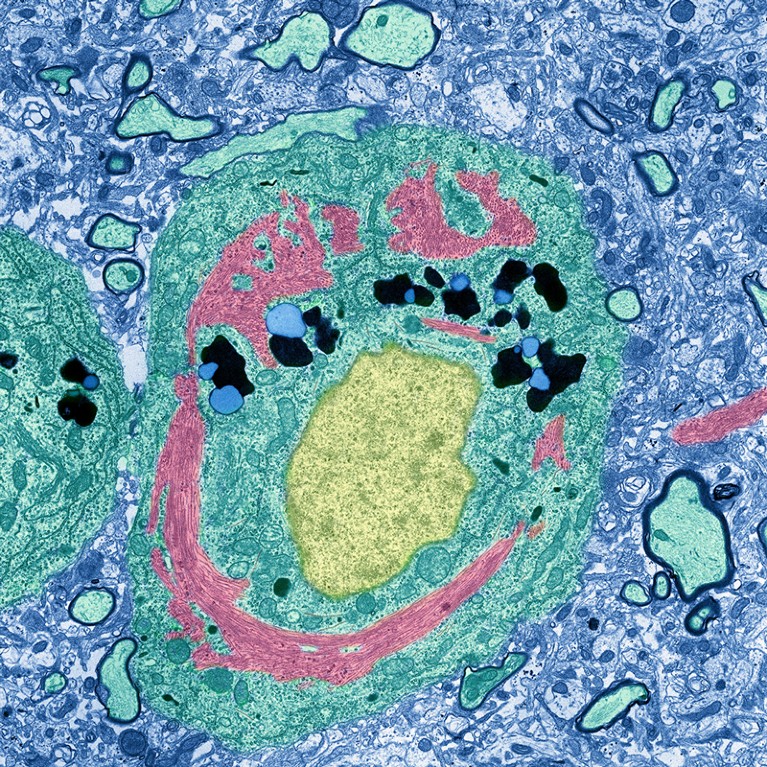 A neuron (green) from a person with Alzheimer’s disease includes an unusual protein complex (pink) encircling the nucleus (yellow).Credit: Thomas Deerinck, NCMIR/SPL
A neuron (green) from a person with Alzheimer’s disease includes an unusual protein complex (pink) encircling the nucleus (yellow).Credit: Thomas Deerinck, NCMIR/SPL
No gene variant is a bigger risk factor for Alzheimer’s disease than one called APOE4. But exactly how the gene spurs brain damage has been a mystery.
A study1 has now linked APOE4 with faulty cholesterol processing in the brain, which in turn leads to defects in the insulating sheaths that surround nerve fibres and facilitate their electrical activity. Preliminary results hint that these changes could cause memory and learning deficits. And the work suggests that drugs that restore the brain’s cholesterol processing could treat the disease.
“This fits in with the picture that cholesterol needs to be in the right place,” says Gregory Thatcher, a chemical biologist at the University of Arizona in Tucson.
Insipid lipids
Inheriting a single copy of APOE4 raises the risk of developing Alzheimer’s around 3-fold; having two copies boosts the chances 8- to 12-fold. Interactions between the protein encoded by APOE4 and sticky plaques of amyloid — a substance tied to brain cell death — in the brain partially explain the connection. But those interactions are not the whole story.
As neuroscientist Li-Huei Tsai at the Massachusetts Institute of Technology (MIT) in Cambridge and her colleagues report today in Nature, APOE4 triggers insulation-making brain cells known as oligodendrocytes to accumulate the fatty molecule cholesterol — a type of lipid — in all the wrong places.
This interferes with the cells’ ability to cover nerve fibres in a protective wrapper made of a lipid-rich material called myelin. Electrical signalling in the brain then slows, and cognition usually suffers.
A hand in a blue glove holding a microscope slide showing four blue blobs.
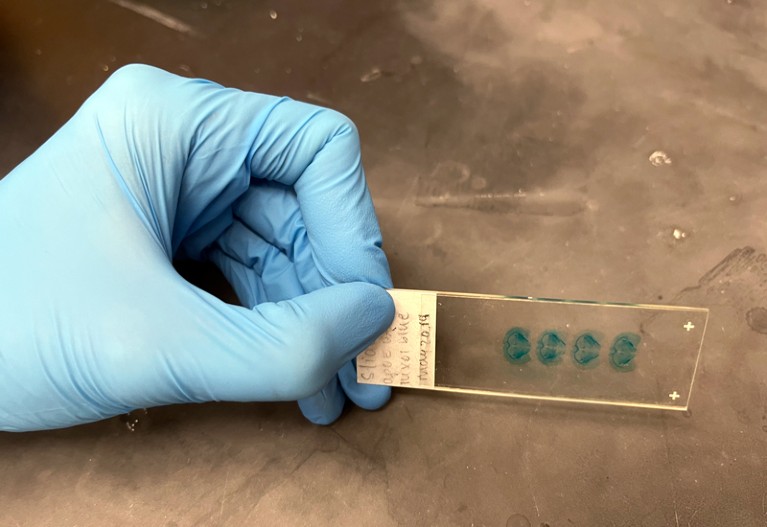 A blue dye shows lipid levels in mouse brain samples: two (left) from mice with a genetic variant called APOE3 and two (right) from mice with a variant called APOE4, which in humans is associated with Alzheimer’s disease.Credit: Elie Dolgin
A blue dye shows lipid levels in mouse brain samples: two (left) from mice with a genetic variant called APOE3 and two (right) from mice with a variant called APOE4, which in humans is associated with Alzheimer’s disease.Credit: Elie Dolgin
Tsai’s team had previously linked lipid changes to malfunctions in other cell types, including some that offer structural support to neurons2 and others that provide immune protection for the brain3. The latest findings add oligodendrocytes and their essential myelin function to the mix.
“It’s really pulling all the pieces together,” says Julia TCW, a neuroscientist at Boston University in Massachusetts.
Cholesterol traffic jam
Working with MIT computational biologist Manolis Kellis, Tsai and her colleagues started by analysing gene activity patterns in tissue from the prefrontal cortex — the brain’s cognitive centre — of 32 deceased people who had two, one or no copies of APOE4 and a range of Alzheimer’s histories.
When the researchers examined APOE4-affected brain cells, they noted abnormalities in many systems for metabolizing lipids. But defects in how oligodendrocytes processed cholesterol seemed “particularly severe”, Tsai says.
The team created cultures of human oligodendrocytes with various forms of the APOE gene. Cells with the APOE4 variant, the group found, tended to hoard cholesterol inside internal organelles. They expelled relatively low amounts of cholesterol, which made them less adept at forming myelin sheaths.
The researchers then treated APOE4-carrying cells with the drug cyclodextrin, which stimulates cholesterol removal. This helped to restore myelin formation. The researchers also found that in mice with two copies of APOE4, cyclodextrin seemed to flush cholesterol out of the brain, improve the flow of cholesterol into myelin sheaths and boost the animals’ cognitive performance.
Cholesterol buster
The mouse findings dovetail with the experience of a person with Alzheimer’s who took a similar formulation of cyclodextrin under a special drug-access programme, as reported in 2020 by the drug’s manufacturer, Cyclo Therapeutics in Gainesville, Florida. The individual’s cognitive functions remained stable over 18 months of treatment, the company says.
However, cyclodextrin might not be ideal for correcting lipid imbalances in the brain. “It’s kind of sledgehammer,” says Leyla Akay, a neuroscientist in Tsai’s lab and a co-author of the latest study. “It just depletes cholesterol from cells.”
But better therapies could emerge now that Tsai and her team have helped to put cholesterol dysregulation on the Alzheimer’s research map. “This study highlights the importance of cholesterol in the brain,” says Irina Pikuleva, a biochemist at Case Western Reserve University in Cleveland, Ohio, “and we now need to try all available strategies to target brain cholesterol.”
Publication: Matheus B. Victor, et al., Lipid accumulation induced by APOE4 impairs microglial surveillance of neuronal-network activity, Cell Stem Cell (2022). DOI: 10.1016/j.stem.2022.07.005
Original Story Source: Massachusetts Institute of Technology

 Alerts Sign-up
Alerts Sign-up