New Drug Delivery Method Harnesses Clotting To Target Anti-Cancer Drugs At Tumors
University of Wisconsin–Madison researchers have developed a new method for targeting tumors with cancer drugs by exploiting the clotting propensity of blood platelets.
University of Wisconsin–Madison researchers have developed a new method for targeting tumors with cancer drugs by exploiting the clotting propensity of blood platelets.
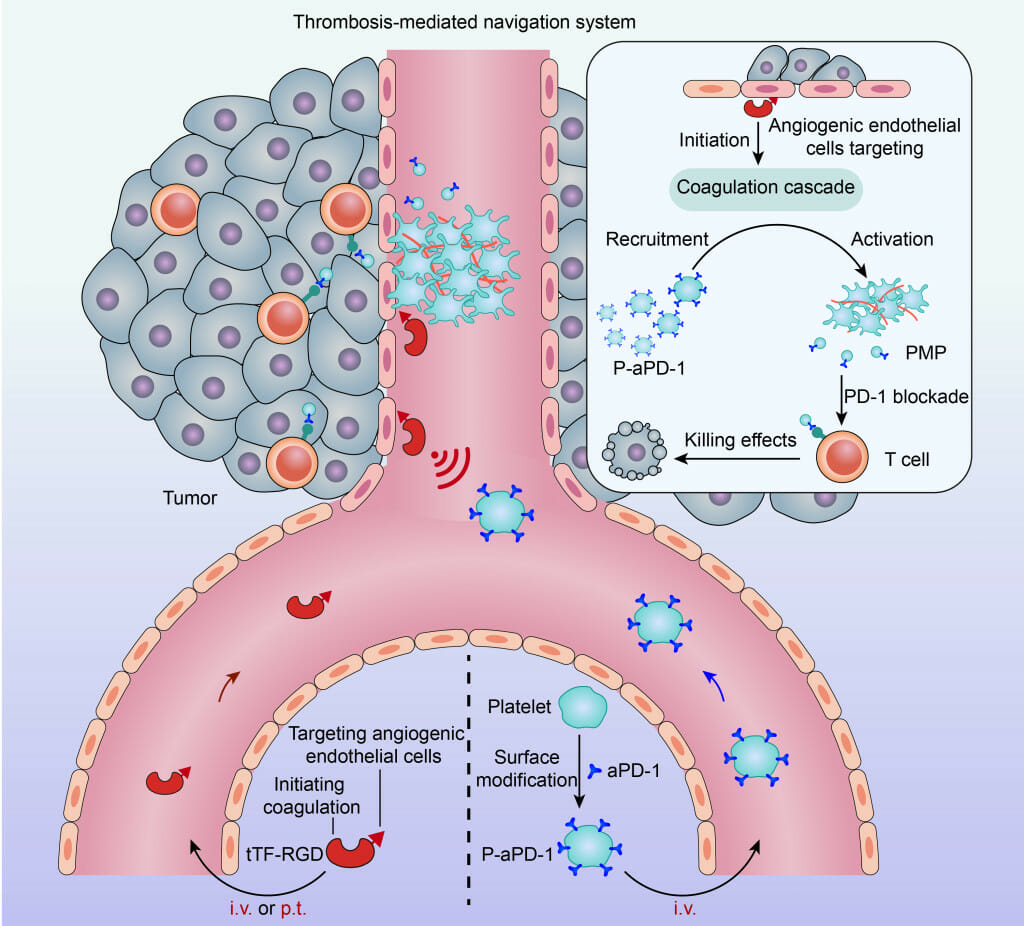
This schematic shows a two-step drug delivery approach to treating cancer tumors developed by UW–Madison researchers. On the left, engineered proteins (shown as red) initiate clotting at the site of a tumor before nanoparticles coated with a blood platelet membrane and loaded with an anti-cancer drug are injected and attracted to the clots within the tumor. IMAGE BY QUANYIN HU
The new approach, first described March 29 in the journal Science Advances, adds to a growing set of innovative drug delivery techniques under development in the lab of Quanyin Hu, a professor in the UW–Madison School of Pharmacy.
One of Hu’s research goals is to improve the effectiveness and safety of cancer immunotherapies. Such treatments have shown promise by bolstering the ability of the immune system to combat cancer cells, but they come with their own set of challenges. One significant obstacle is that the drugs can target normal cells in addition to tumors. This can make the treatments less effective and sometimes lead to serious side effects.
 Quanyin Hu
Quanyin Hu
To better target the therapeutics at tumors, Hu and his colleagues considered a mechanism that results in a very specific biological chain reaction — the cellular signals that trigger blood to clot.
“This whole study is purely inspired by nature and the natural features of platelets to participate in clot formation,” says Hu.
First, the researchers used an engineered protein designed to locate and bind to tumor blood vessels and then initiate thrombosis, or clotting, within tumors. They found that in mice that received the engineered proteins via intravenous injections, the proteins led to clot formation almost exclusively within tumors, with only very limited thrombosis occurring elsewhere.
The clot formation is not the therapy itself, but rather creates conditions for efficient, targeted drug delivery to the clot-filled tumor, which Hu describes as a “cellular hive.”
“Once we have this clot formation at the tumor site, we have this so-called cellular hive that can attract these therapeutic drones,” Hu says.
In this case, the drones are blood platelets engineered with immunotherapeutics on their surfaces which the researchers inject once clotting has begun.
In their studies using mice, Hu and his colleagues found that the engineered platelets efficiently delivered a common immunotherapy drug known as an immune checkpoint inhibitor to the tumor sites. The checkpoint inhibitors encourage immune cells known as T cells to eradicate cancer cells.
Mouse models with colorectal tumors that received the treatment saw their tumors shrink and lived longer than mice that received a traditional immunotherapy treatment. Notably, one-third of the mice that received the treatment became completely tumor free.
The researchers then tested the delivery method with blood platelet derivatives — nanoparticles coated with a platelet membrane and loaded with a chemotherapy drug — on mouse models with human breast cancer tumors. Similarly, they found that the chemotherapy drugs were efficiently delivered to tumor sites while avoiding normal cells. The findings open the possibility to more effective and versatile cancer treatments with multiple types of drugs and fewer unintended side effects.
Before the delivery system can go to clinical trials, more testing is required to ensure its safety, especially with respect to clot formation. While their studies in mice have thus far shown that the system creates clots only in tumor sites, a more comprehensive safety evaluation is the next step, Hu says. That process will likely take a couple years.
Publication: Wang, Y., et al. Active recruitment of anti–PD-1–conjugated platelets through tumor-selective thrombosis for enhanced anticancer immunotherapy. Science Advances, (2023). DOI: 10.1126/sciadv.adf6854
Original Story Source: University of Wisconsin–Madison

 Alerts Sign-up
Alerts Sign-up