Natural Gas Could Bridge Gap From Gasoline To Electric Vehicles, Thanks To Metal-Organic Frameworks
As the world turns its attention to electric vehicles as a replacement for gas-powered cars and trucks, some vehicles such as long-haul trucks and planes will need a bridge between gas and electric.
As the world turns its attention to electric vehicles as a replacement for gas-powered cars and trucks, some vehicles such as long-haul trucks and planes will need a bridge between gas and electric.
Natural gas could be a viable alternative. It’s widely available and burns more cleanly than gasoline. There are even conversion kits already available to allow your passenger cars or long-haul trucks to run on natural gas, says Adam Matzger, professor of chemistry at the University of Michigan.
“Natural gas is everywhere, and it’s seen as sort of a stepping stone fuel from gasoline to electric or hydrogen,” he said. “The main problem with it is storage. Cost is good. Distribution is good. Storage is the problem.”
Matzger, who studies a material called metal-organic frameworks, thought that these MOFs had untapped potential to store methane, the largest component of natural gas.
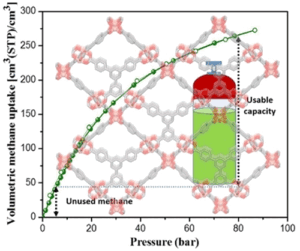 Promising MOFs were identified computationally and experimentally demonstrate remarkable methane uptake that outperforms known benchmarks both volumetrically and gravimetrically. Advanced set of interatomic potentials that explicitly accounts for the presence of coordinatively unsaturated sites (CUS) in MOFs were used to identify the high-capacity MOFs that were previously overlooked due to the limitation of the general interatomic potentials. Image credit: Angewandte Chemie
Promising MOFs were identified computationally and experimentally demonstrate remarkable methane uptake that outperforms known benchmarks both volumetrically and gravimetrically. Advanced set of interatomic potentials that explicitly accounts for the presence of coordinatively unsaturated sites (CUS) in MOFs were used to identify the high-capacity MOFs that were previously overlooked due to the limitation of the general interatomic potentials. Image credit: Angewandte Chemie
MOFs are rigid, porous structures composed of metals linked by organic ligands. Methane can be stored within an MOF through a process called adsorption. In adsorption, the molecules of a substance cling to the surface of a material making storage at low pressures possible.
Matzger worked with Alauddin Ahmed, assistant research scientist in mechanical engineering at the U-M College of Engineering, to scan nearly one million MOFs that have already been developed to find materials that might have the right characteristics to store methane. They found two that had not been previously tested, one of which had coincidentally been created in Matzger’s lab. Their results are published in Angewandte Chemie, a journal of the German Chemical Society.
The problem with natural gas is that it needs to be stored under very high pressure, or about 700 times atmospheric pressure. Storing natural gas under this kind of pressure requires specialized equipment and a large amount of energy.
“There’s one other little wrinkle, which is if you’re actually going to use it in a vehicle, you’re not going to take it from the high pressure and bring it down to zero,” Matzger said. “Because when the pressure gets too low, you can’t operate the vehicle’s engine. So you actually need to look at usable capacity.”
To make methane usable, scientists needed to figure out the best material that would both store methane at a lower pressure, but which can also cycle it up to the level of pressure needed by the vehicle’s engine. That meant cycling between 80 times atmospheric pressure to about five times atmospheric pressure.
“The idea is by having an adsorbent in a tank, you can store more methane at lower pressures than you could without the absorbent because it helps to hang on to the methane at lower pressures,” Matzger said. “So then the problem comes down to picking an adsorbent, and this is where the theory really came to the rescue.”
Assistant research scientist Ahmed specializes in developing algorithms to predict properties of chemical compounds and nanoporous materials—materials such as MOFs that have the capability of storing molecules—and using computational screening to identify particular nanoporous materials. He developed a method to screen a master database of the 1,000,000 MOFs that he compiled from 21 different databases.
“Why this material is important is because from a chemistry point of view, you can design an infinite number of these MOFs,” Ahmed said. “So the question is, if the number is infinite, how do you find a good material? It’s kind of like finding a needle in a haystack—actually, it’s harder than that.”
Ahmed used two different methods to screen for two different classes of MOFs. One class of MOFs have what’s called a closed metal site. Another other class of MOFs have an open metal site, but only once the researchers computationally scrubbed water molecules from within the structure of these MOFs.
The U-M researchers were able to search for MOFs with an open metal side—more inviting to methane molecules—based on an algorithm developed by Don Siegel, professor of mechanical engineering at the University of Texas.
“Previously, when researchers searched for MOFs to store methane, they didn’t separate these two classes of MOFs,” Ahmed said. “The advantage of our modeling is we have two separate models. We separated those compounds with closed metal sites from those with open metal sites, which have a greater affinity for those methane molecules.”
The team turned up three MOFs that would work well to store methane, one of which Matzger’s lab had coincidentally developed. Postdoctoral fellow Karabi Nath was able to synthesize the materials with high surface area and found their experimental methane capacities matched what theory had predicted. The MOFs, UTSA-76, UMCM-152 and DUT-23-Cu, work well because they have many small pores that can attract gas molecules inside.
Matzger envisions a tank inside of a truck filled with these MOFs. Currently, cars and trucks converted to run on natural gas use expensive tanks designed to store gas under 10,000 pounds per square inch, or PSI. Instead, drivers could use a lower pressure tank filled with UMCM-152 or one of the two other MOFs identified.
“The thing that sets this study apart is that we set the record for methane storage. These MOFs are better than any other methane storage material previously identified, and so that helps us figure out whether we’re getting close to a practical system,” Matzger said.
“But the thing that just keeps making me laugh is that one of the ideal MOFs was right under our noses and we didn’t know it. That’s where the theory, no question, set us in the right direction.”
Publication: Dr. Karabi Nath, et al., Computational Identification and Experimental Demonstration of High-Performance Methane Sorbents, Chemical Society (2022). DOI: 10.1002/anie.202203575.
Original Story Source: University of Michigan

 Alerts Sign-up
Alerts Sign-up