Artificial Intelligence Provides New Insight into Preventing Human Disease
A molecular machine, which plays an essential ‘cargo’ role in controlling the delivery of proteins to the surface of human cells, and is implicated in several diseases, has been identified in a landmark study using artificial intelligence (AI).
A molecular machine, which plays an essential ‘cargo’ role in controlling the delivery of proteins to the surface of human cells, and is implicated in several diseases, has been identified in a landmark study using artificial intelligence (AI). The research, led by an international team of scientists, is published today [11 May] in Cell.
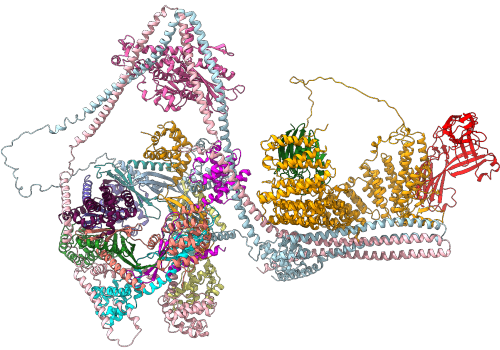 Illustration of the Commander Function.
Illustration of the Commander Function.
Researchers at the Universities of Bristol and Queensland (Australia), and the Medical Research Council Laboratory of Molecular Biology in Cambridge, sought to understand how Commander, a molecular machine composed of sixteen individual proteins, is assembled and how mutations in its function play a role in Ritscher-Schinzel syndrome, a rare disease characterised by intellectual disability and development delay.
Professor Pete Cullen, one of the study's lead authors from the School of Biochemistry at the University of Bristol, explained: "The surface of every human cell is studded with an array of proteins through which they engage with their neighbours and sense their environment. How these proteins reach the cell surface in the right amount and at the right time is fundamental to understanding human development and the process of healthy human aging."
By combining biochemical and cell-based experiments with state-of-the-art artificial intelligence the researchers were able to precisely define how individual parts of Commander come together to form the functional machine and how this acts as one of the cell's 'postal workers'.
Professor Brett Collins from The University of Queensland's Institute for Molecular Bioscience and one of the study’s lead authors, said: "Just as the postal system has processes to transport and sort cargo, so cells in our body have molecular machines that transport and sort proteins. Cargo transport is all about getting the right parcels to the right destination at the right time – in cells, the Commander complex controls this system to ensure the right amount of protein is delivered to the right place in the cell."
Professor Collins added: "Mutations in the Commander complex have been shown to disrupt the transport of lipids into cells, causing people with Ritscher-Schinzel syndrome to suffer with high cholesterol and heart defects."
Knowing the structure of the Commander complex has allowed the researchers to better understand how disease-causing mutations provoke Commander to malfunction in Ritscher-Schinzel syndrome and advance our understanding of how it is involved in other diseases. For example, viruses such as SARS-CoV-2, which causes COVID-19, and human papilloma virus (HPV), which can lead to cancer, need the Commander complex to infect cells. Commander has also been linked to the transport of the amyloid protein in Alzheimer’s Disease.
Professor Cullen said: "Excitingly, we now have a blueprint for thinking of ways that we can manipulate Commander function to help with these and other diseases associated with defects in the fascinating molecular machine."
The researchers concluded: "Finding the complete structure of Commander would not have been possible even two years ago without these new advances in AI technologies."
The research was funded by the National Health and Medical Research Council (Australia), in the UK by the Medical Research Council, Wellcome Trust, The Royal Society, and the EndoConnect European Research Training Network.
Publication: Michael D. Healy, et al., Structure of the endosomal Commander complex linked to Ritscher-Schinzel syndrome, Cell (2023). DOI: 10.1016/j.cell.2023.04.003.
Original Story Source: University of Bristol

 Alerts Sign-up
Alerts Sign-up