New Research from UC San Diego Sheds Light on the Possible Origins of Life
Discovery of functional prebiotic metabolism shows promise for improving carbon-capture technologies
Researchers at the University of California San Diego have identified the conditions for cell metabolism to emerge on the early Earth, shedding new light on the origins of life itself, along with the fundamental nature of biological carbon fixation.
“Notably, this advance can be used to design and develop novel carbon capture methods,” said UC San Diego bioengineering professor Bernhard Palsson, the Principal Investigator on the study.
“Moving toward a circular carbon economy, the mathematical models and computational tools that resulted from this study will be useful for the development of future C1-carbon technologies, paving the way for the design of economical biomanufacturing systems with a minimal carbon footprint,” said Amir Akbari, the lead scientist of the study and a data scientist in the Shu Chien-Gene Lay Department of Bioengineering at the UC San Diego Jacobs School of Engineering.
The paper, "Metabolic homeostasis and growth in abiotic cells," appears in the May 1, 2023 issue of the Proceedings of the National Academy of Sciences (PNAS).
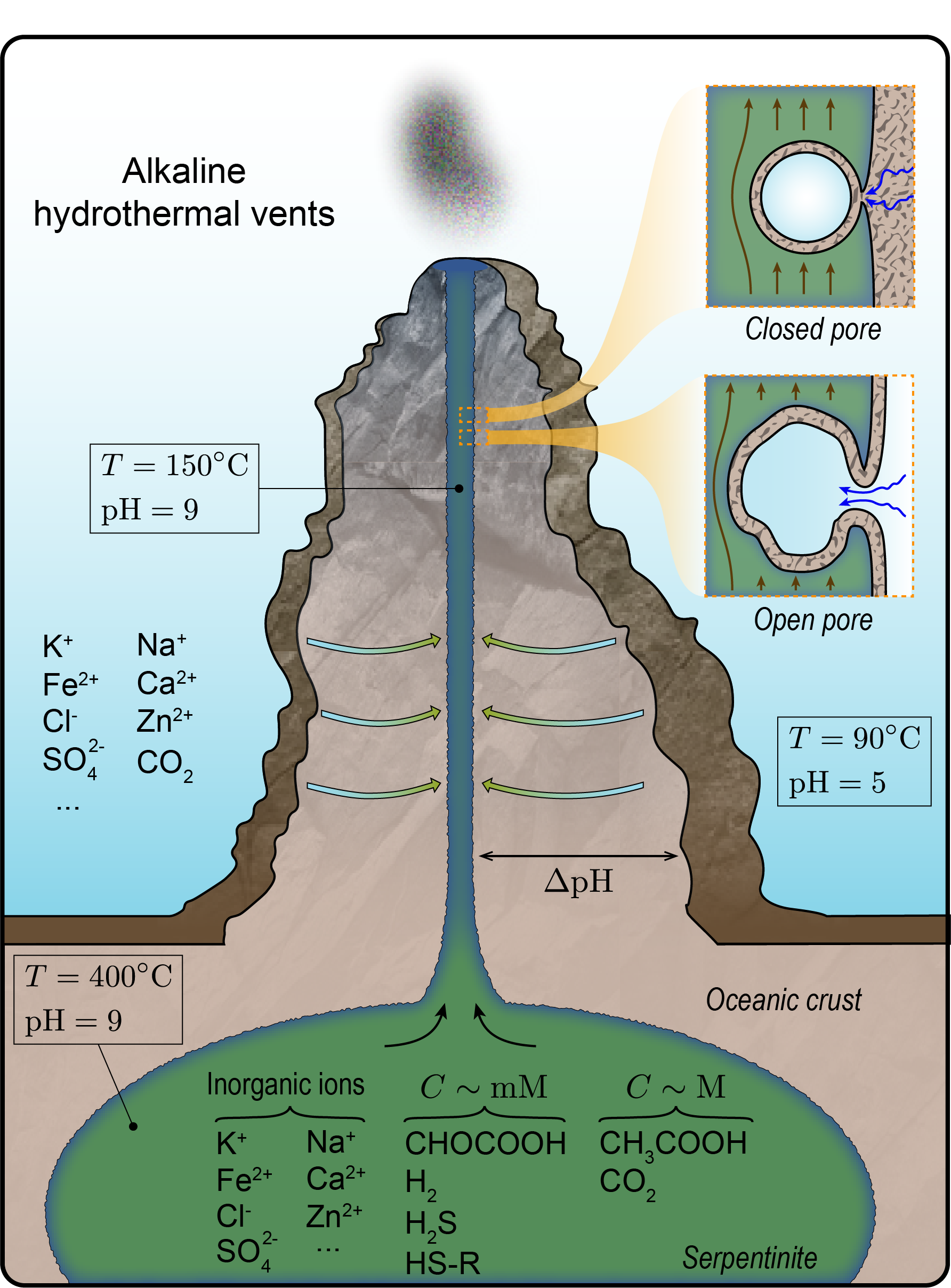
 Submarine alkaline vents are areas on the ocean floor where hydrothermal vents release hot, mineral-rich fluids. Photo credit: D. Kelley/ M. Elend/UW/URI-IAO/NOAA/The Lost City Science Team.
Submarine alkaline vents are areas on the ocean floor where hydrothermal vents release hot, mineral-rich fluids. Photo credit: D. Kelley/ M. Elend/UW/URI-IAO/NOAA/The Lost City Science Team.
Background: The pathways to the origins of life
Metabolic pathways are the series of chemical reactions that cells use to convert nutrients into energy and other molecules. These pathways are thought to have played an important role in the emergence of life on earth, as they allow cells to utilize energy efficiently and create complex molecules, which are essential for life.
One such metabolic pathway is carbon fixation, which is the process by which carbon dioxide is converted into complex carbon-based molecules that could be used by living cells. Researchers have long studied the origins of this process in search of fundamental insights into improving carbon capture technologies.
But how did such a process emerge? To find answers, UC San Diego researchers looked to one of the places on Earth where the first carbon fixing cycles are thought to have occurred: submarine alkaline vents.
Submarine alkaline vents are areas on the ocean floor where hydrothermal vents release hot, mineral-rich fluids. These vents provide an environment that is conducive to the formation of complex molecules, such as proteins and lipids. This is thought to have been an important factor in the origin of life, as it provided an environment that was rich in the necessary ingredients for life to emerge.
In this study, researchers examined the possibility that the first carbon fixing cycles emerged in alkaline hydrothermal vent environments.
“Currently, there is no consensus in the origins-of-life community as to how the first metabolic networks emerged and operated on the early Earth and how they evolved into more complex, self-sustaining chemical reaction networks,” Akbari said. “In the metabolism-first paradigm, which our paper falls into, the assumption is that early metabolic pathways operated nonenzymatically, only relying on inorganic catalyst/energy source/reducing agents and simple carbon sources that were likely available on the early Earth.”
 Discovery of functional prebiotic metabolism shows promise for improving carbon-capture technologies
Discovery of functional prebiotic metabolism shows promise for improving carbon-capture technologies
The birth of a new theory
Research conducted over the last decade has provided clues as to what first metabolic pathways might have looked like, demonstrating that some metabolic-like reactions can proceed nonenzymatically under plausible prebiotic conditions. However, it remains unknown whether all these reactions can spontaneously occur under the same conditions and using the same reagents, or if they necessarily cooperate in a confined environment. Thus, there remains a significant gap between showing the feasibility of individual steps and demonstrating the possibility that different steps can self-organize into self-sustaining chemical reaction networks.
“One of the main results of the paper is demonstrating that the underlying mechanism for why first carbon fixing cycles could only work in a narrow parameter range from first principles, linking it to energy requirement constraints and difficulty of selectively concentrating the organic products of these cycles, which were necessary for the evolution of complex metabolic pathways in abiotic compartments available on the early Earth,” Akbari said. “We also demonstrated that both these issues were linked to the membrane potential, implying that the membrane potential was as essential to the emergence of life at its origin as it is to all modern living systems
Next steps
The cycles presented in the team’s research are both “self-sustaining,” meaning they can continue to operate and consistently reproduce their chemical products using nutrients and energy sources that are available in their environments without outside intervention, and “self-amplifying,” meaning they strengthen themselves as they progress. This opens avenues of research that are of particular interest within the origins-of-life field.
“With regard to the specific topic of this paper, the next step is to demonstrate that carbon fixing cycles such as these can be reproduced in the lab non-enzymatically using purely inorganic catalysts, energy sources and reducing agents, which could have existed on the early Earth,” said Akbari. “I believe there are several research groups in the origins-of-life field who are currently pursuing this line of research.”
In the bigger picture, one of the long-term objectives of the origins-of-life field is to create artificial life in the lab. More specifically, the goal is to recreate all the steps required to transition from a simple inorganic reaction system to a complex biochemical reaction network capable of undergoing Darwinian evolution. The researchers say that this study may prove to be a step in that direction.
Paper: “Metabolic homeostasis and growth in abiotic cells.” Authors: Amir Akbari, Department of Bioengineering, University of California San Diego; and Bernhard O. Palsson, Department of Bioengineering, UC San Diego, and Novo Nordisk Foundation Center for Biosustainability, Technical University of Denmark.
This work was funded by the Novo Nordisk Foundation (Grant Number NNF10CC1016517) and the National Institutes of Health (Grant Number GM057089)
Publication: Amir Akbari, et al., Metabolic homeostasis and growth in abiotic cells, PNAS (2023) DOI: 10.1073/pnas.2300687120
Original Story Source: UC San Diego

 Alerts Sign-up
Alerts Sign-up